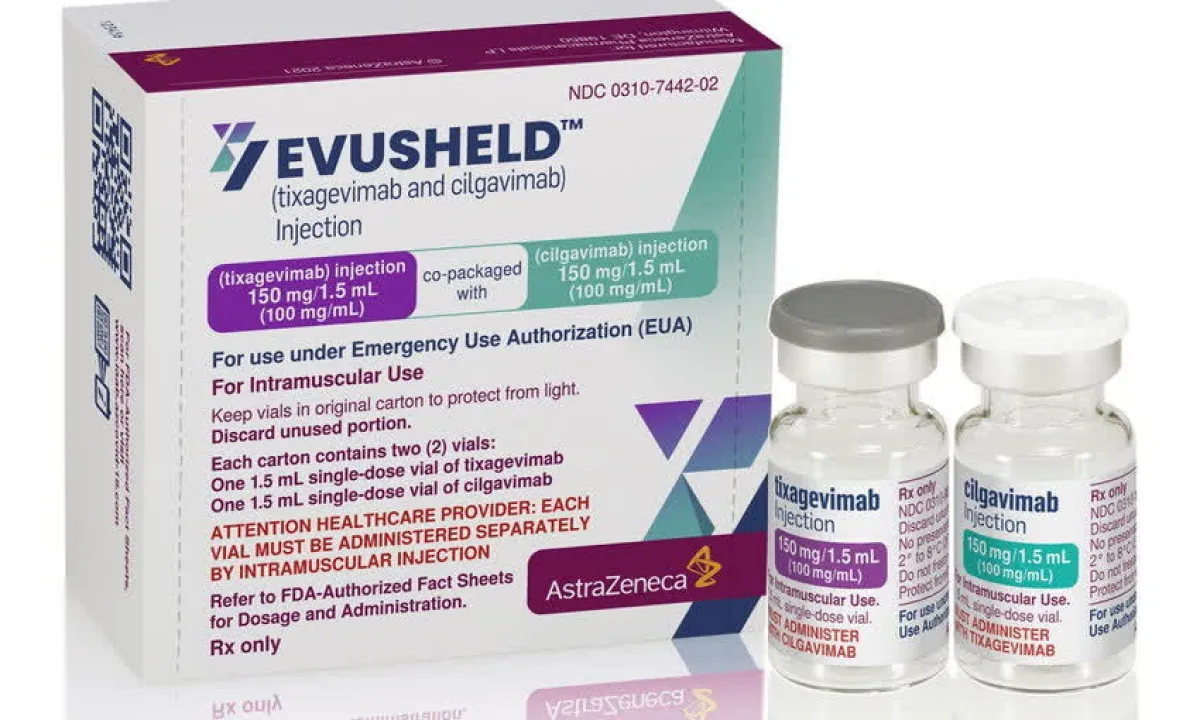
Evusheld injections can now be used to treat coronavirus disease 2019 (COVID-19). The drug will help reduce severe illness and death from COVID-19 infection by 50-60%.
The Food and Drug Administration (FDA) approved the registration of AstraZeneca Co., Ltd., for Evusheld under a conditional authorization in emergency situations on 27 June 2022, for use in the prevention of COVID-19. And most recently, Evusheld has been added to the indication as an injection to treat COVID-19 for people with mild to moderate symptoms. In adults and adolescents aged 12 years and over with a body weight of at least 40 kilograms, a dose of 600 milligrams originally approved for use to prevent COVID-19.
People should receive Evusheld as soon as possible when the results of COVID-19 infection are known and should receive the drug within seven days after symptom onset.
People who need to use this drug should provide preliminary information to the doctor, such as a history of drug allergies, history of cardiovascular disease treatment, pregnancy, breastfeeding, and any other health conditions.
Source: Food and Drug Administration Office, 88/24 Tiwanon Road, Talad Khwan Subdistrict, Mueang District, Nonthaburi Province 11000.
Telephone: +66 2590 7000
1 November 2022